Specification system for moulds on cotton
Kaese M.1, Meyer, G.1, Schneider, T.2, Weidenboerner M.3
1 Hanse Analytik GmbH, Fahrenheitstr. 1, 28359 Bremen, Germany
2 Faserinstitut Bremen (FIBRE), Wachtstr. 17-24, 28195 Bremen, Germany
3 Institut für angewandte Mikrobiologie, Universität Gießen, Senckenbergstr. 3, 35390 Gießen, Germany
Abstract: Raw cotton from different provenaences was examined for contamination with moulds. 27 species, predominantly of the genera Aspergillus, Eurotium and Penicillium were identified and classified by literature studies with regard to their potential for fibre degradation and their health implications. The physically explainable coherences between mould contamination and fibre degradation were experimentally justified. We developed a quantitative and qualitative specification system for moulds on cotton to be used by technical engineers without specialised knowledge in mycology. This system consists of a filter-module for the isolation of the fungal spores, which can be received as an additional element for a marketable trashtester, and of a catalogue for the specification of moulds which imparts the necessary microbiological knowledge.
Introduction
1. Mould contamination of cotton
Many moulds can be found on cotton. Depending on the degree of cotton fibre processing different mould species can be isolated. The so-called field-fungi can be found on the cotton plant, especially the open bolls, the fibres and seeds. These are among other things parasitical or plant-pathogenic species, which are able to degrade the fibres before harvest (21, 24). After harvest, storage-fungi can be isolated. These species are quite tolerant of dryness or lack of oxygen and are spread all over the world in most cases. Climatic factors can lead to the dominance of a few species of this spectrum of moulds on cotton (8, 20). At least damp cotton textiles are a good substrate for the so-called material deteriorators. Their growth leads to spots and holes in the textile. These species are storage-fungi with high demands on humidity (14,16). The growth of moulds on cotton can be detected under light- or electron-microscopic observations as visible filamentous mycelia, conidia and asco- or sporangio-spores. The germ cells are still detectable if the growth of moulds is impossible due to unfavourable environmental conditions. These permanent forms make it possible to analyse the species of moulds that contaminate cotton fibres by cultivation on culture media for fungi in retrospect.
2. Health implications of moulds
Moulds can lead to infections, predominantly of the lung and skin, chronic poisoning and allergies (13, 17). The risk of an infection by mould-contaminated cotton or of poisoning by the inhalation of spores containing mycotoxins is very low for consumers of cotton products. On the one hand, only facultative pathogen species can be found on cotton, but on the other, great amounts of spores or fibres containing mycotoxins exceeding the critical dose must be inhaled. There is a higher risk for people who are professionally exposed. That is one reason why precautions in the cotton industry should be taken and why they should get better (10, 19).
Allergies resulting from to the contact with moulds or their spores occur in the whole population. Mycoallergoses are found more frequently amongst people who are professionally exposed to increased amounts of mould spores in the air. This has been the case in agriculture and food processing, but it has not been proved for cotton processing up to now. The amount of mould spores releasing a primary sensitiveness (comparatively high amount) and a secondary allergic reaction (comparatively low amount) differs from species to species and from human to human (2, 10). Also that the use of mould-contaminated cotton in upholstered furniture, mattresses or futons results in allergic reactions to sensitive persons has not been proved up until now, either.
3. Moulds inducing cotton fibre degradation (incl. pre-experiment)
Cotton fibres contain 94% cellulose in their dry mass. Degradation of the cellulose leads to the complete loss of structure and strength of the fibres. Cellulose degradation is a natural process performed by micro-organisms. They produce low molecular cellulolytic substances and a multitude of extra-cellular enzymes called cellulases which cooperate in the transformation of cellulose to monomer glucose. Moulds dispose of cellulases in very different preportions. Usually the cellulolysis is induceable, meaning that fungi produce cellulases only if there are suitable substrates in their environment (1, 6, 14, 23). High performance strains of the fungi Trichoderma reesei and Aspergillus niger are used for the biotechnological production of cellulases in big reactors (12). Many fungi spread all over the world and are common on raw cotton, at least as spores. Favourable growing conditions are useful for fibre degradation by moulds. These conditions - high temperature, high humidity, low content of oxygen - are frequently present during storage and transport of raw cotton. In this connection, easily satisfied fungi can increase humidity and temperature by their growth. This promotes the development of more demanding species (17).
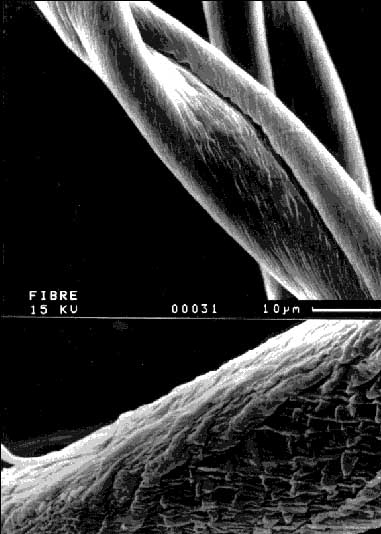
The degree of fibre degradation by mould contamination was experimentally determined. Raw cotton from the provenaence South Africa was sterilised and inoculated with 7 different fungi. All attempts including one not inoculated control were incubated in water or in a culture medium at 25 oC for 14 days. The cotton fibres were dried and their strength was measured with a stelometer. The fibre strength was 20,3 g/tex before the experiment. The loss of strength in the not inoculated control was about 25%. The loss of strength in the attempts with the moulds was up to a further 38,5% (according to the control). In some cases there was no loss of strength, however. Data are shown in Table 1. We could demonstrate that the incubation of cotton with moulds leads to a loss of fibre strength that differs according to the species of fungi. The nutrients that are available to the fungi influence the degree of fibre degradation in a species-specific manner. Each species has a characteristic potential to produce cellulolytic enzymes, depending on its genetic equipment and the prevailing environmental conditions. We could demonstrate in electron microscopic examinations that a fibre degradation resulting from the growth of moulds is the reason for the loss in fibre strength. Figure 1 shows undamaged cotton fibres from the control attempt and a REM-picture of partially degraded fibres from an incubation experiment. The degradation by cellulases from the mould Rhizopus stolonifer is recognisable on the surface structure: the cuticula bursts and the degradation process starts on the cellulose fibrils underneath. The crystalline, high ordered structure of cotton cellulose is transformed into an amorphous structure, which has a much lower tensile strength (7, 23).
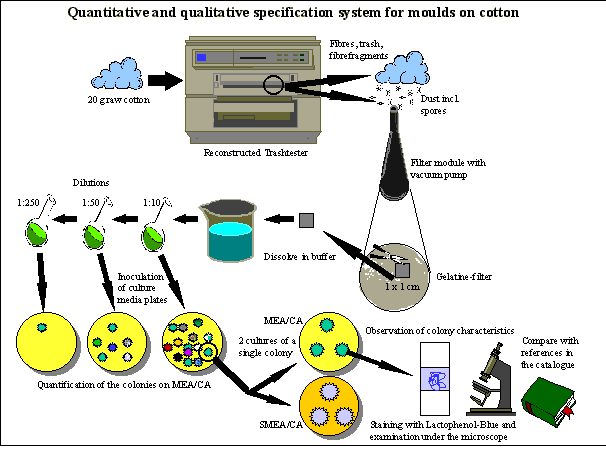
Development of a specification system for moulds on cotton (see figure 2)
1. Reconstruction of the trashtester Hollingworth TT-2000 with a vacuum by-pass and a gelatine-filter-module
The mould germ cells are removed from the cotton fibres in a trashtester. Raw cotton is divided into the four fractions pure fibres, trash (rests of plants and seeds), fibre-fragments and dust. The trashtester is routinely used for determining the purity of raw cotton. The mould spores are separated from the dust by a filter-module outside the reconstructed trashtester. For reconstruction, you drill a hole into the front of the dust-drawer of the apparatus and fix a connecting piece above the dust-filter. A tube connects this piece with a filter-module and a vacuum pump. The filter-module contains a gelatine-membrane-filter with a pore width of 3 µm (Sartorius SM 12602, Germany). The vacuum pump produces a constant aerosol-flow of 20 l/min in this by-pass through the filter-membrane. The by-pass does not affect the examination of the cotton in the trashtester and yields a representative sample of mould germ cells in relation to 20 g raw cotton. Additional elements to modify the dust-drawer of the marketable trashtester Hollingworth TT-2000 can be obtained at FIBRE (see the address above).
2. Quantification of the mould contamination
A 1 cm2 piece of the gelatine-filter-membrane from the filter-module of the trashtester is cut out and dissolved in 3 ml Phospate-NaCl-Peptone-buffer to determine the amount of germinable mould spores and mycelia-fragments. This suspension is diluted 1:10, 1:50 and 1:250 in the same buffer. Aliquots of 300 µl of each dilution are used to inoculate MEA/CA-plates. It is a culture medium for fungi containing malt-extract and chloramphenicol (30 mg/l) to suppress the growth of bacteria. The plates are incubated for 3 to 14 days at 25 oC. Then the number of colonies per plate, resp. per dilution degree is established and the number of colony-forming-units in the spore-suspension and per cm2 gelatine-filter is calculated. It does not make sense to calculate a value for the whole bale of cotton, because the amount of 20 g raw cotton used for the examination is not a representative sample. However, it is possible to compare cotton from different provenaences with regard to its mould contamination if test conditions are identical and if a large number of tests is performed.
3. Specification of the moulds and their classification with respect to potential fibre degradation and health implications on the basis of the fungi catalogue
Single colonies on the MEA/CA plates that appear in a different way are used to inoculate one MEA/CA-plate and one SMEA/CA plate (high salinity) with a three-point-inoculation. This is done for all distinctive colonies. On these culture media each species shows a more or less characteristic growth of the colonies after 1 or 2, or a maximum of 4 weeks of incubation at 25 oC. A sample of each colony is stained with Lactophenol-blue on a slide and examined under a microscope with an enlargement of 20x up to 1000x. You compare the observations of the colony-morphology on the different media and the examination under the microscope with the references in the catalogue ``Moulds on Cotton" that will contain pictures and detailed descriptions of about 25 mould species found on cotton. The catalogue will be available at Hanse Analytik GmbH (see address above) . For supplementary information the moulds can be examined under an electron microscope. A small piece of a colony is punched out with the culture medium, dried above silica gel and placed on a sample slide for the REM. The sample is fumed with gold and an REM-picture is taken. The catalogue ``Moulds on Cotton" contains corresponding pictures as references.
Results and discussion
1. Quantification of the mould germ cells contamination on cotton from 6 provenaences
The amount of germ cells isolated from raw cotton from 6 provenaences was determined and compared with the stickiness of the fibres, measured with a honey-dew-tester GRAF SCT and with the fluorescence of the fibres at UV 365 nm. Data are shown in Table 2. The mould contamination increased in the following order: South Africa < Arizona < Australia < Greece < Chad < Pakistan. Cotton from the provenaence Pakistan was contaminated 26,5 times more than cotton from South Africa. The stickiness of the fibres, resp. the degree of honey-dew contamination did not correlate to the mould contamination. The observation of fluorescence under UV365 is also not a suitable test for the quantification of the mould contamination.
2. Specification and characterisation of mould genera and species isolated from raw cotton
The contamination of raw cotton from 10 provenaences with moulds was qualitatively analysed by specification. The genera and species are shown in Table 3. Only examination of further samples from several harvest years will show if there is a characteristic spectrum of species for each provenaence. The large part of identified species belongs to the nearly related genera Aspergillus, Eurotium and Penicillium and are typical storage-fungi. We conclude that the contamination of raw cotton took place during and after the harvest and was a result of improper storage. Among the isolated fungi are the species Aspergillus flavus, A. niger and Rhizopus stolonifer, which are known as cellulase producing fungi. These fungi are able to degrade cotton fibres. The isolated species A. flavus, A. niger and Penicillium chrysogenum can produce dangerous mycotoxins and may lead to allergic reactions and other health impairments (1, 3, 19). The mould species isolated from raw cotton are nearly identical with the species that were found on cotton fibres or in the air of cotton mills in Great Britain some years ago (9, 11). We conclude from these data, that raw cotton is regularly contaminated with the mould genera Aspergillus, Cladosporium, Eurotium, Penicillium and Rhizopus, and with bacteria of the genus Streptomyces. In high probability, increased amounts of mould spores in indoor air at sites where raw cotton is treated mechanically can be proved.
3. Benefits from the specification system for moulds on cotton
Damaged fibres owing to mould contamination of cotton lead to processing problems, reduced strength and irregular stainability of the yarn and the textile. This results in interruptions during the production process and loss of product quality, resulting in economic losses (4, 22). Damages in textiles often become visible and there are complaints after long-term use. To obtain an expert´s opinion on damaged, reclaimed articles retrospectively in order to find out what happened and who was liable (supplier, manufacturer or ready-to-use trader) is more expensive than the previous examination of the raw cotton with the specification system. The usual quick tests like the honey-dew-test or the observation of UV fluorescence do not give any information concerning quantity and possible results of contamination of cotton with moulds. The specification system for moulds on cotton permits a simple quantitative and qualitative characterisation of contamination before cotton processing.
Another aspect is the improvement of product quality by examining and certifying the health safety of the product. On the one hand, it becomes more and more popular to fill upholstered furniture, mattresses and futons with untreated natural fibres, but on the other, allergic diseases resulting from unknown indoor agents are becoming more problematic (19). This suggests better examination of the raw material cotton. In the interest of consumers´ protection, especially cotton fibres used to fill upholstery or pillows for living-rooms and bedrooms should without any doubt be free of mould contaminations.
4. Subsequent examinations
Examination of further samples from several harvest years will supply enough data to demonstrate if there is a coherence between the spectrum of species and the amount of moulds and cotton provenaence. Including data about climatic, storage- and transport-conditions of the cotton, we hope to get a detailed understanding on why there is mould contamination and to develop techniques to avoid it.
Tables and figures
Tab. 1:
Strength reduction of cotton fibres released by experimental contamination with moulds.Stelometric measurement, n per species = 10.
Tab. 2: Contamination with moulds and honeydew on cotton from 6 provenaences. (n) = number of experiments
Tab. 3: Mould species and genera found on raw cotton, their distribution to provenaences and important species-specific characteristics (relating to references 3, 5, 8, 11, 15, 18, 20). (x) = shown in the catalogue (prototype), * = no mould. Abbreviations: F. on cot. = Found on cotton up to now, Fibre deg. = Potentially Fibre-degradation, Health inj. = Potentially injurious to health, T = Produces Toxins, A = Allergenic, M = Releases Mycoses
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fig. 1: Electron microscopic detection of the fibre degradation induced by mould contamination. Upper picture: Undamaged raw cotton, Provenaence South Africa. Underneath: The same cotton after 14 days incubation with Rhizopus stolonifer in culture medium. Visible change of the surface structure by enzymatic degradation of cellulose.
Fig. 2: Schematic description of the specification system for moulds on cotton. Abbreviations: MEA = Malt-Extract-Agar, SMEA = Saline-Malt-Extract-Agar, CA = Chloramphenicol. References 1. Akhmedova, Z.R., Beltskaya, O.P., Dalimova, G.N., Khalikova, M.M. Azimkhodzhaeva, M.N., Davranov, K.D., Sharipova, A. (1994): Selection and cultivation of cellulose and lignin degrading fungi. Microbiology 63, 523-527
2. Bunse, T., Merk, H. (1992): Mycological aspects of inhalative mould allergies. Mycoses 35, 61-66
3. Domsch, K.H., Gams, W., Anderson, T.H. (1980): Compendium of soil fungi. Academic Press Inc. New York
4. Doraiswamy, I., Chellamani, P. (1991): Making the most of indian cottons. Textile Asia 22, 101-103
5. Frisvad, J.C., Samson, R.A. (1991): Filamentous fungi in foods and feeds, ecology, spoilage and mycotoxin production. In: Arora, D.K., Mukerij, K.D., Marth, E.H. (Ed.): Handbook of applied Mycology, Vol. 3, Foods and Feeds. Marcel Dekker Inc. New York Basel Hong Kong
6. Ghosh, B.K., Ghosh, A. (1992): Degradation of cellulose by fungal cellulase. In: Winkelmann, G. (Ed.): Microbial degradation of natural products. VCH Verlag Weinheim New York
7. Hoshino, E., Sasaki, Y., Mori, K., Okazaki, M., Nisizawa, K., Kanda, T. (1993): Electron microscopic observation of cotton cellulose degradation by exo- and endo-type cellulases from Irpex lacteus. J Biochem 114, 236-245
8. Kozakiewicz, Z. (1989): Aspergillus species on stored products. Mycological papers 161. CAB International
9. Lacey, J. (1977): Microorganisms in air of cotton mills. The Lancet Aug/1977, 455-456
10. Lacey, J. (1991): Aerobiology and health, the role of airborne fungal spores in respiratory disease. In: Hawksworth, D.L. (Ed.): Frontiers in mycology, 4th mycological Congress Regensburg 1990. CAB International
11. Lacey, J., Lacey, M.E. (1987): Microorganisms in the air of cotton mills. Am Occup Hyg 31, 1-19
12. Lowe, D.A. (1992): Fungal enzymes. In: Arora, D.K., Mukerji, K.G., Marth, E.H. (Ed.): Handbook of applied Mycolgy Vol. 4, Fungal Biotechnolgy. Marcel Dekker Inc. New York Basel Hong Kong
13. Male O. (1991): The significance of mycology in medicine. In: Hawkswort, D.L. (Ed.): Frontiers in mycology, 4th mycological Congress Regensburg 1990. CAB International
14. Montegut, D., Indictor, N., Koestler, R.J. (1991): Fungal deterioration of cellulosic textiles, a review. International Biodeterioration 28, 209-226
15. Raper, K.B., Fennel, D.I. (1965): The genus Aspergillus. Williams and Wilkins Company Baltimore
16. Reiß, J. (1983): Materialzerstörung durch Schimmelpilze. Chemische Rundschau 36, Nr. 39, 1-7
17. Reiß, J. (1986): Schimmelpilze. Springer Verlag, Berlin/Heidelberg
18. Roth, L., Frank, H., Kormann, K. (1990): Giftpilze, Pilzgifte, Schimmelpilze, Mykotoxine. Ecomed Verlagsgesellschaft Landsberg/Lech
19. Samson, R.A., Flannigan, B., Flannigan, M.E., Verhoeff, A.P., Adan, O., Hoekstra, E.S. (1994): Health implications of fungi in indoor environments. Air quality monographs, Vol. 2. Elsevier Amsterdam
20. Samson, R.A., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O. (1995): Introduction to food-borne fungi, 4th Edition. Centaalbureau voor Schimmelcultures Baarn/Delft
21. Simpson, M.E., Marsh, P.B., Merola, G.V., Ferretti, R.J., Filsinger, E.C. (1973): Fungi that infect cottonseeds before harvest. Applied Microbiology 26, 608-613
22. Spinnereileitung Hch. Kettelhack, Rheine, Deutschland (1994): Personal communication
23. Vaheri, M.P. (1982): Oxidation as a part of degradation of crystalline cellulose by Trichoderma reesei. Journal of applied Biochemistry 4, 356-363
24. Watkins, G.M. (1993): Compendium of cotton diseases, 2nd Edition. The American Phytopathological Society